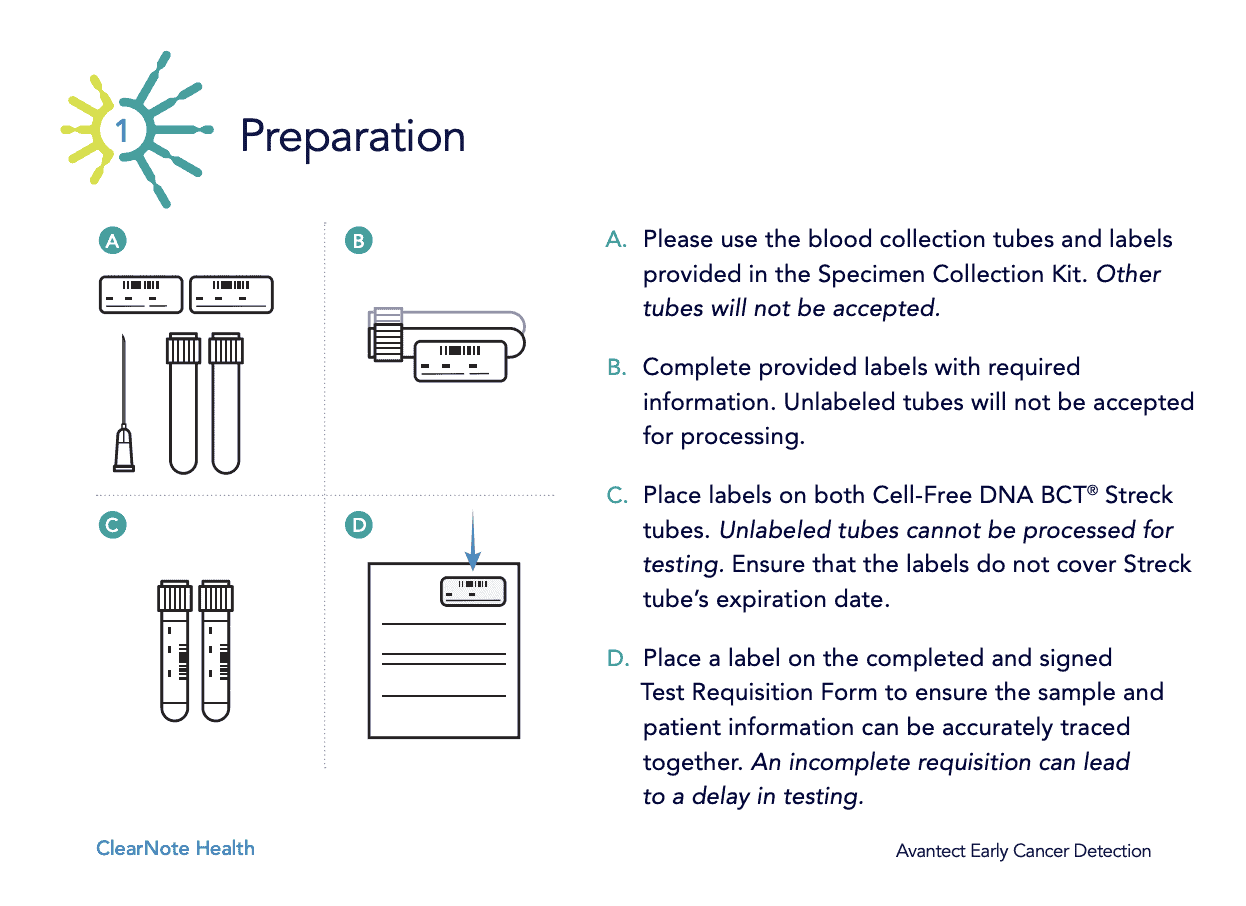
Using Avantect In Your Practice
Using Avantect In Your Practice
The Avantect Pancreatic Cancer Test allows you to detect pancreatic cancer early in high-risk patients such as those aged ≥50 with newly diagnosed type 2 diabetes and those with a genetic predisposition and/or family history of pancreatic cancer.
Easy to implement pancreatic cancer testing
The Avantect test is easily incorporated into clinical management protocols for high-risk patients. A simple blood draw can deliver life-changing information.
Clear, easy to interpret pancreatic cancer test results
“Abnormal Epigenomic Signal was DETECTED” means that an abnormal signal was found in the patient’s sample, and that pancreatic cancer may be present. It is recommended that communication of a positive test result take place in a setting that includes appropriate counseling as well as clinical and diagnostic follow-up.
“Abnormal Epigenomic Signal was NOT DETECTED” means that an abnormal signal was not found when the patient’s sample was compared with the thousands of samples at ClearNote Health. This is not a definitive sign that cancer is not present, as there is the possibility for false-negative results.
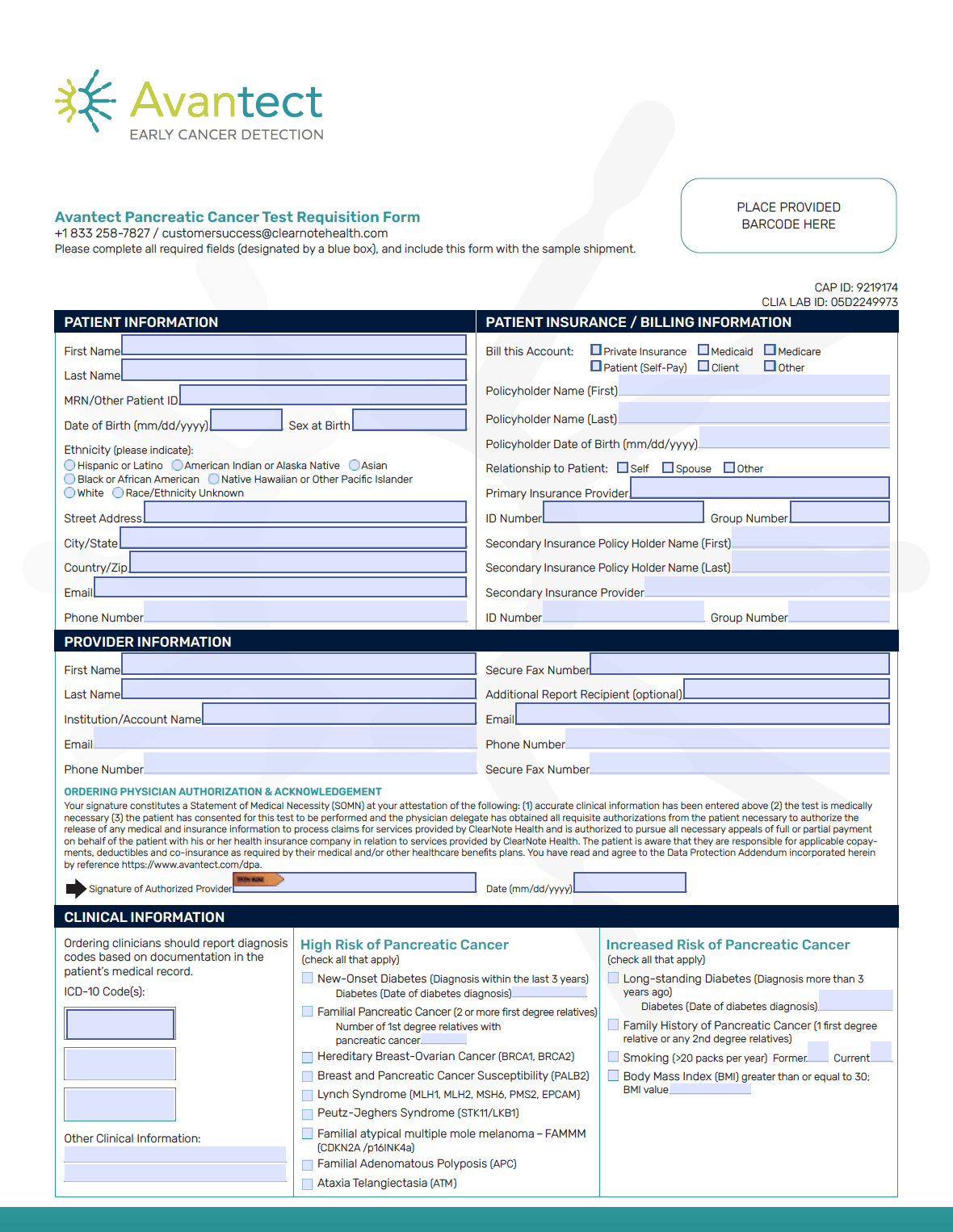
Test Forms
Participate in the Avantect Physician Experience Program
The Physician Experience Program is a unique way to introduce the Avantect Pancreatic Cancer Test to your practice. This program provides an opportunity for qualifying practices to gain experience with the Avantect test with eligible patients over age 50 who are newly diagnosed with type 2 diabetes.
- To get started, a representative from ClearNote Health will explain how the program works, help you set up an account, and send you specimen collection kits.
- Once you’ve joined the program, your feedback will be important to help us continue to improve the ordering experience.
Avantect Patient Access Program
Our Customer Success Team will review coverage options and offer support based on each patient’s needs. For patient-specific information regarding out-of-pocket costs, including copays, coinsurance, and deductibles, please call our Customer Success Team at +1 833-258-7827.
ClearNote Health believes that health equity begins with equal access to patient care across all patient communities. We support those who are uninsured or underinsured, and we offer a discounted price to patients choosing a self-pay option. Financial assistance will be offered through a quick, confidential, over-the-phone, income-based needs assessment.
Our Customer Success Team will review coverage options and offer support based on each patient’s needs. For patient-specific information regarding out-of-pocket costs, including copays, coinsurance, and deductibles, please call our Customer Success Team at +1 833-258-7827.
Good-faith estimates
Uninsured patient financial assistance
HSAs and FSAs accepted
Self-pay patient rate
Individualized payment plans
Important information
The Avantect Pancreatic Cancer Test is an early detection test. The test does not establish a diagnosis of pancreatic cancer, and results should be considered in the context of other clinical criteria. Definitive diagnosis of pancreatic cancer usually requires a series of imaging scans, blood tests, and a biopsy. Not all pancreatic cancers will be detected. Some patients with pancreatic cancer may have a “Signal not detected” result. Some patients without pancreatic cancer may have a “Signal detected” result. False-negative and false-positive results are possible. A “Signal not detected” result does not guarantee that no pancreatic cancer is present. In some cases, no result is obtained. While this is very uncommon, it may be caused by shipping delays or when there is not enough cell-free DNA for the test in the patient’s blood. If this happens, we generally ask for a repeat blood sample for testing at no extra cost.
The test was developed in the ClearNote Health CLIA-certified (CLIA# 05D2249973) and CAP-accredited (CAP# 9219174) laboratory and has not been cleared or approved by the US Food and Drug Administration (FDA).
References and notes
- ClearNote Health, data on file.