Order the
Avantect Test
Order The Avantect Test
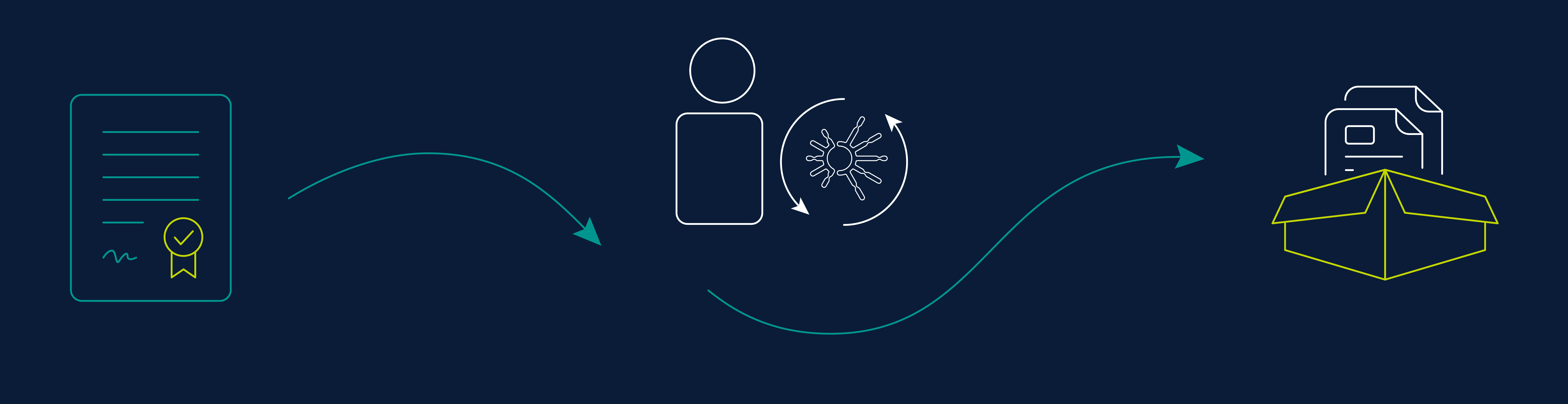
1) Submit Account Set-Up Form
Fill out the account set-up form below. We will contact you within 24 hours.
2) Request Test Kits
We will confirm your requirements and dispatch your test kits.
3) Receive Test Kits Your kits will ship the next business day and usually arrive in 2-3 business days.
Offer early cancer detection in as fast as
4 business days
1) Submit Account Set-Up Form
Fill out the account set-up form below. We will contact you within 24 hours.
2) Request Test Kits
We will confirm your requirements and dispatch your test kits.
3) Receive Test Kits Your kits will ship the next business day and usually arrive in 2-3 business days.
Provider Account Form
The Avantect test kits can be requested by qualified healthcare professionals who have a provider account with ClearNote Health. If you are a patient interested in information about this test, please contact your primary care physician or submit an enquiry via our general contact form. We typically reply within one working day.
Important information
The Avantect Pancreatic Cancer Test is an early detection test. The test does not establish a diagnosis of pancreatic cancer, and results should be considered in the context of other clinical criteria. Definitive diagnosis of pancreatic cancer usually requires a series of imaging scans, blood tests, and a biopsy. Not all pancreatic cancers will be detected. Some patients with pancreatic cancer may have a “Signal not detected” result. Some patients without pancreatic cancer may have a “Signal detected” result. False-negative and false-positive results are possible. A “Signal not detected” result does not guarantee that no pancreatic cancer is present. In some cases, no result is obtained. While this is very uncommon, it may be caused by shipping delays or when there is not enough cell-free DNA for the test in the patient’s blood. If this happens, we generally ask for a repeat blood sample for testing at no extra cost.
The Avantect Ovarian Cancer Test is an early detection test. The test does not establish a diagnosis of ovarian cancer, and results should be considered in the context of other clinical criteria. Definitive diagnosis of ovarian cancer usually requires a series of imaging scans, blood tests, and a biopsy. Not all ovarian cancers will be detected. Some patients with ovarian cancer may have a “Signal not detected” result. Some patients without ovarian cancer may have a “Signal detected” result. False-negative and false-positive results are possible. A “Signal not detected” result does not guarantee that no ovarian cancer is present. In some cases, no result is obtained. While this is very uncommon, it may be caused by shipping delays or when there is not enough cell-free DNA for the test in the patient’s blood. If this happens, we generally ask for a repeat blood sample for testing at no extra cost.
The tests were developed in the ClearNote Health CLIA-certified (CLIA# 05D2249973) and CAP-accredited (CAP# 9219174) laboratory and have not been cleared or approved by the US Food and Drug Administration (FDA).