Avantect is a blood test that detects epigenomic and genomic profiles of cell-free DNA (cfDNA) associated with pancreatic cancer. Developed using cutting-edge epigenomic and genomic science as well as machine learning, it provides actionable information to help guide next steps when a cancer signal is detected. Watch our educational video to learn more.
The Avantect Pancreatic Cancer Test is specifically designed for the early detection of pancreatic cancer
A routine blood draw makes it easy to incorporate the Avantect test into clinical management protocols for high-risk patients
Clear information about whether signals are detected, with recommendations for next steps
Epigenomic changes are present in the earliest stages of pancreatic cancer,1 and 5-hydroxymethylcytosine (5hmC), a chemical modification of DNA, can be used as a targeted biomarker to identify these changes.
Using the latest epigenomic and genomic science, the Avantect test may reveal the emergence of cancer cells early.2,3 Its streamlined technology uses a combination of 5hmC profiling and whole-genome sequencing in a single assay to enable a deep look into cancer biology.1
See how easy it is to use the Avantect test with your high-risk patients.

The Avantect test has been validated in patients at a high risk for developing pancreatic cancer, including those aged 50 and older who are newly diagnosed with type 2 diabetes and those with a genetic predisposition and/or family history of pancreatic cancer. The test’s performance has an overall sensitivity of 66.7% and specificity of 96.9%.3
The Avantect test report makes it easy to find the information you need to guide decisions about potential next steps for any additional diagnostic procedures.
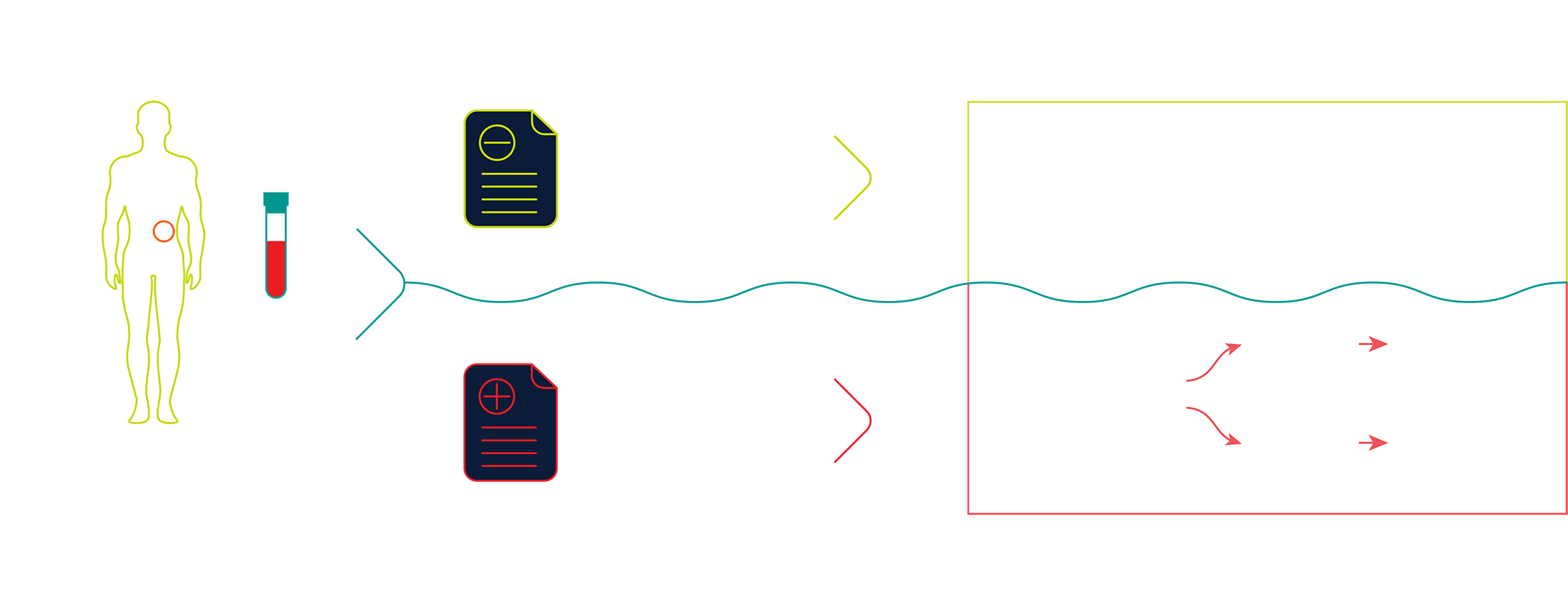
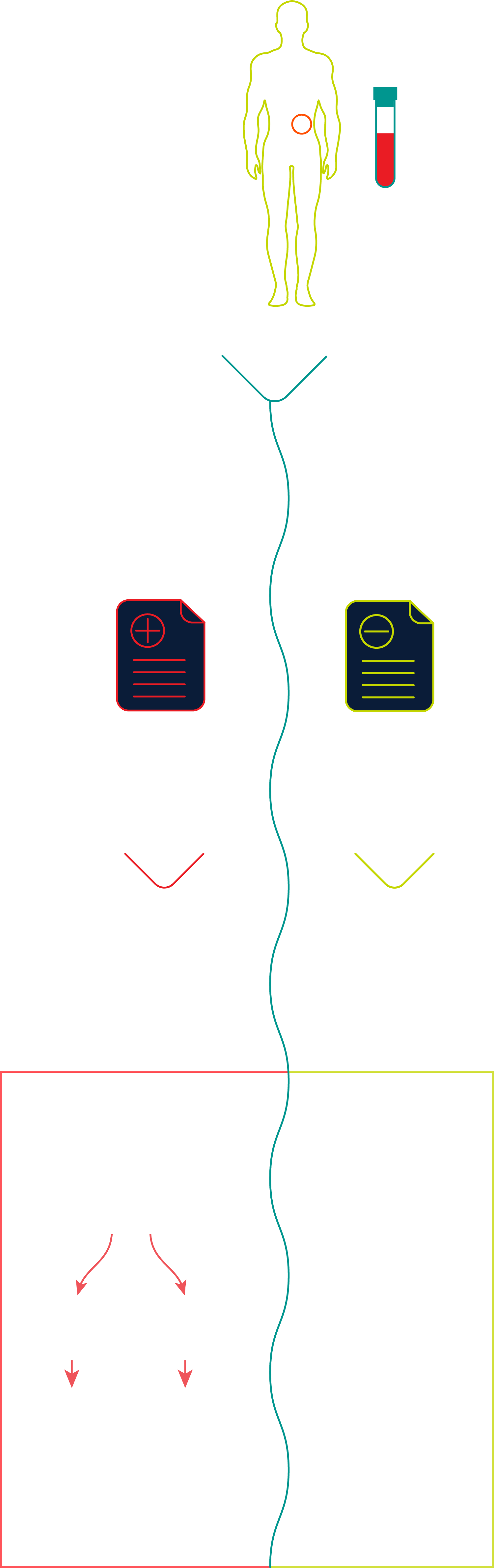
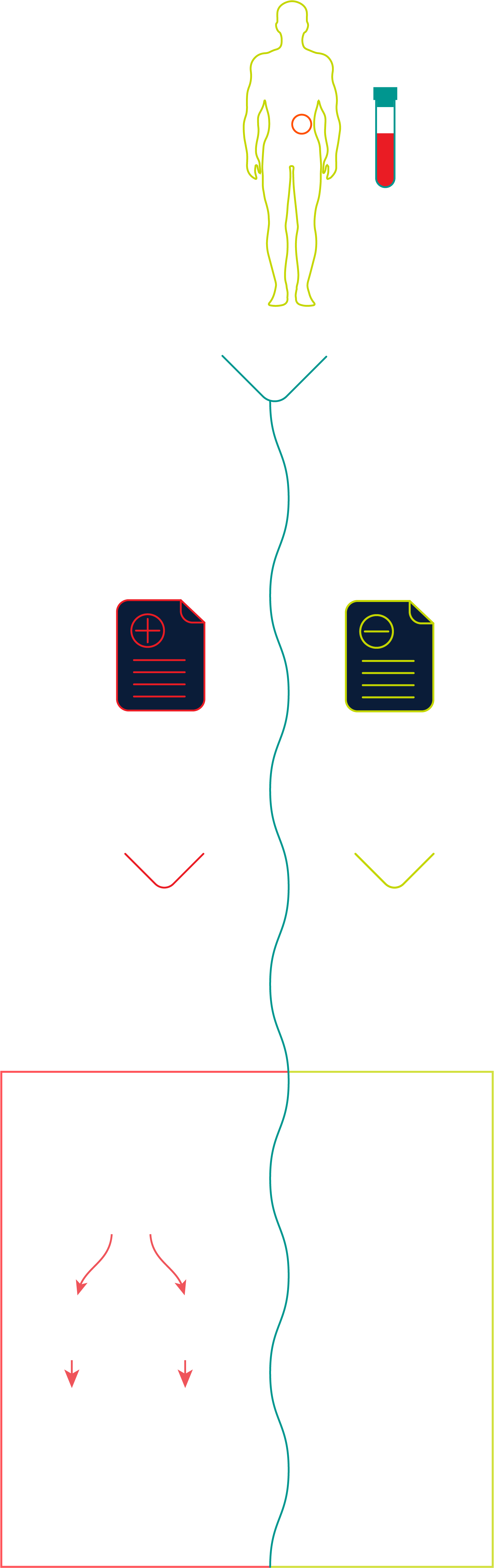
For patients with a genetic predisposition and/or family history of pancreatic cancer who receive an Avantect Pancreatic Cancer test result, standard guidelines for imaging protocols by leading professional societies such as the AGA (American Gastroenterological Association) and, the NCCN (National Comprehensive Cancer Network) for pancreatic cancer surveillance should be followed if clinically indicated.
* The Avantect test is performed in ClearNote Health’s CLIA-certified and CAP-accredited laboratory.
**Clinical studies to determine the utility of repeat testing after a negative or presumed false-positive result are ongoing.
NOTE: Currently, there are no established guidelines or recommendations for clinical management after cfDNA testing for pancreatic cancer. The above represents suggested protocols and timeframes, but it is up to the discretion of the treating physician to determine further management, including the medical necessity and timing of repeat testing.
The Avantect Pancreatic Cancer Test is an early detection test. The test does not establish a diagnosis of pancreatic cancer, and results should be considered in the context of other clinical criteria. Definitive diagnosis of pancreatic cancer usually requires a series of imaging scans, blood tests, and a biopsy. Not all pancreatic cancers will be detected. Some patients with pancreatic cancer may have a “Signal not detected” result. Some patients without pancreatic cancer may have a “Signal detected” result. False-negative and false-positive results are possible. A “Signal not detected” result does not guarantee that no pancreatic cancer is present. In some cases, no result is obtained. While this is very uncommon, it may be caused by shipping delays or when there is not enough cell-free DNA for the test in the patient’s blood. If this happens, we generally ask for a repeat blood sample for testing at no extra cost.
The test was developed in the ClearNote Health CLIA-certified (CLIA# 05D2249973) and CAP-accredited (CAP# 9219174) laboratory and has not been cleared or approved by the US Food and Drug Administration (FDA).