Avantect Ovarian Cancer Test
Avantect Ovarian Cancer Test
A breakthrough innovation for the early detection of ovarian cancer
The Avantect Ovarian Cancer Test is a novel, cell-free DNA-based blood test to aid in earlier diagnosis in women at high risk for developing ovarian cancer, offering a greater chance for improved survival.
Purpose-built
The Avantect Ovarian Cancer Test is specifically designed for the early detection of ovarian cancer
Easy to use
A routine blood draw makes it easy to incorporate the Avantect test into clinical management protocols
Actionable report
Clear results with recommendations for next steps
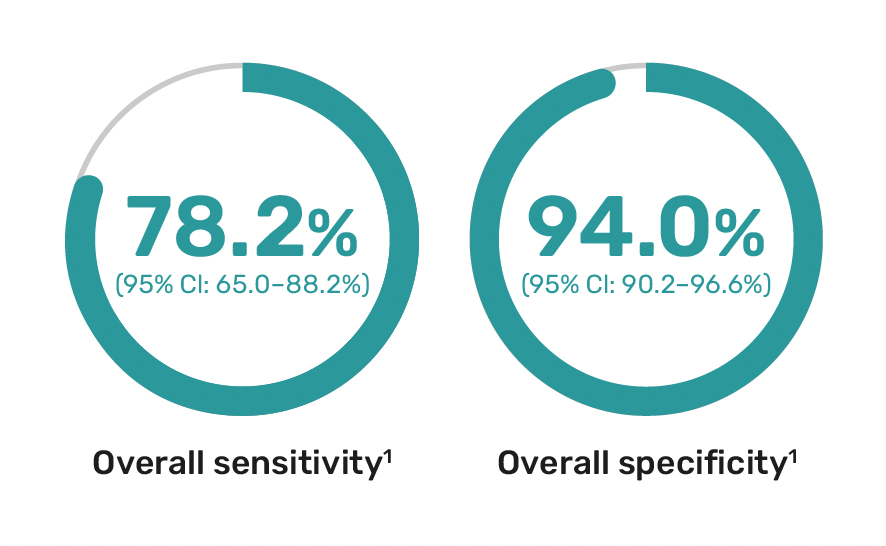
Optimized performance in high-risk patients
The Avantect test has been validated in a study involving over 300 patients, including those at high risk for ovarian cancer.
Easy to Interpret Test Results
The Avantect Ovarian Cancer Test measures the presence or absence of an abnormal signal in cell-free DNA that is associated with ovarian cancer. The Avantect Ovarian Cancer report provides a qualitative result of an “abnormal signal detected” or “abnormal signal not detected.” However, if a signal is detected, an intensive follow-up is recommended, including an oncological evaluation, further imaging studies like TVUS or MRI.

Easy to Order
The Avantect test is easily incorporated into clinical management protocols for high-risk patients. A simple blood draw can deliver life-changing information.
Get started with Avantect
See how easy it is to use the Avantect test with your high-risk patients.

Important information
The Avantect Ovarian Cancer Test is an early detection test. The test does not establish a diagnosis of ovarian cancer, and results should be considered in the context of other clinical criteria. Definitive diagnosis of ovarian cancer usually requires a series of imaging scans, blood tests, and a biopsy. Not all ovarian cancers will be detected. Some patients with ovarian cancer may have a “Signal not detected” result. Some patients without ovarian cancer may have a “Signal detected” result. False-negative and false-positive results are possible. A “Signal not detected” result does not guarantee that no ovarian cancer is present. In some cases, no result is obtained. While this is very uncommon, it may be caused by shipping delays or when there is not enough cell-free DNA for the test in the patient’s blood. If this happens, we generally ask for a repeat blood sample for testing at no extra cost.
The test was developed in the ClearNote Health CLIA-certified (CLIA# 05D2249973) and CAP-accredited (CAP# 9219174) laboratory and has not been cleared or approved by the US Food and Drug Administration (FDA).
References and notes
ClearNote Health, data on file.