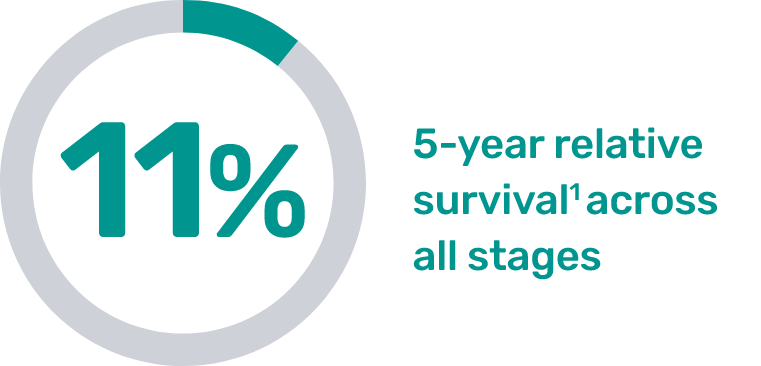
Prioritizing high-risk patients
Pancreatic cancer is called a “silent killer” because it often develops without symptoms.4
It is paramount to identify the patient populations who are at high risk, such as those such with a genetic predisposition, family history, or conditions like newly diagnosed type 2 diabetes, when early detection might make all the difference.3,4