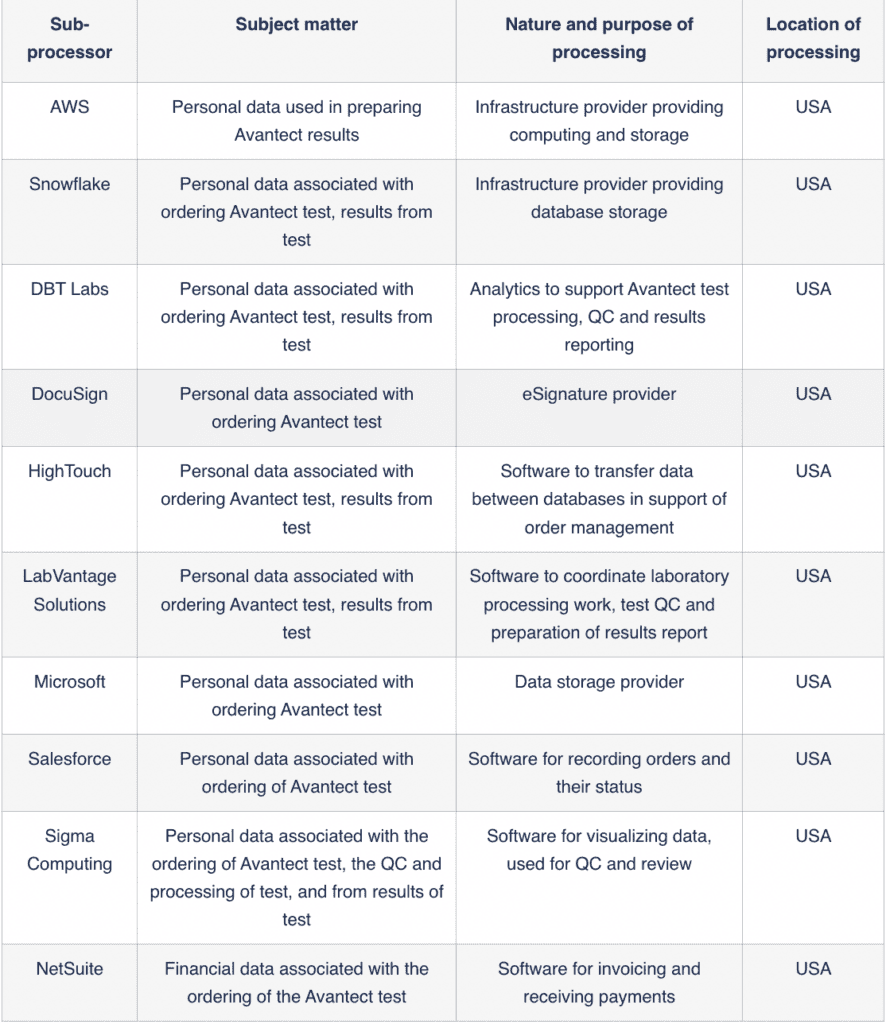
Subprocessor | Subject matter | Nature and purpose of processing | Location of processing |
AWS | Personal data used in preparing Avantect results | Infrastructure provider providing computing and storage | USA |
Snowflake | Personal data associated with ordering Avantect test, results from test | Infrastructure provider providing database storage | USA |
DBT Labs | Personal data associated with ordering Avantect test, results from test | Analytics to support Avantect test processing, QC and results reporting | USA |
DocuSign | Personal data associated with ordering Avantect test | eSignature provider | USA |
HighTouch | Personal data associated with ordering Avantect test, results from test | Software to transfer data between databases in support of order management | USA |
LabVantage Solutions | Personal data associated with ordering Avantect test, results from test | Software to coordinate laboratory processing work, test QC and preparation of results report | USA |
Microsoft | Personal data associated with ordering Avantect test | Data storage provider | USA |
Salesforce | Personal data associated with ordering of Avantect test | Software for recording orders and their status | USA |
Sigma Computing | Personal data associated with the ordering of Avantect test, the QC and processing of test, and from results of test | Software for visualizing data, used for QC and review | USA |
NetSuite | Financial data associated with the ordering of the Avantect test | Software for invoicing and receiving payments | USA |
We use cookies, subject to your consent, to analyze the use of our website and to ensure you get the best experience. Third parties with whom we collaborate can also install cookies in order to show you personalized advertisements on other websites. Read our cookie policy for more information.
Read our privacy policy to understand how ClearNote Health handles personal information that we collect through our digital properties that link to this Privacy Policy, including our website and other activities described in the privacy policy.